We the Translational Cell Biology (TCB) team aims at understanding the biology of developmental and neural plasticity and primary brain tumors. We have developed a new platform for brain tumors and their impact on the tumor microenvironment. The new platform allows to approach the tumor zones. The Tumor Zone model integrates new findings and concepts of cancer biology applable for clinical medicine.
Based on the heterogenous Tumor Zones we follow three strategies:
I. Unravelling the composition of the tumor microenvironment. Uncovering the metabolic Achilles‘ heel of brain tumors and identifying the metabofitness key parameters‘ in glioma patients.
II. Developing intra-operative imaging techniques such as the DiVA and vascular DiVA for supra-complete tumor resection.
III. Understanding neural regeneration and plasticity.
I. Brain tumor microenvironment in the limelight
Understanding the biology of malignant brain tumors (called gliomas) is still challenging in the neuroscience field. Although long time viewed as a homogeneous cell mass with uncontrolled proliferation, brain tumors show remarkable cellular complexity bearing a sort of stem cell properties allowing them to differentiate into vessels, glial cells and even neurons in vitro. To reveal in particular how brain tumor cells shape their microenvironment and thereby escaping immune control our research focuses on tumor released factors or the secretome of brain tumor cells. One major research line of our group is the study of secreted factors derived from gliomas with neurotoxic and gliotoxic potential. Glioma cells are genetically pluripotent and thus share many features with stem cells. In particular, gliomas possess many metabolic programs at an immature state thereby creating an advantage growth niche floating the microenvironment with secreted metabolites (Fig. 1). The neurotransmitter glutamate is a key factor involved in glioma-induced neurodegeneration (Savaskan et al., 2008). However, the biology of glioma-derived secreted factors appears pleiotropic, i.e. affecting microglia, astrocytes as well as neurons (Savaskan et al., 2010). There is strong evidence that transporters for energy metabolites take center stage in this process (Savaskan, 2010).
*FIGURE
Fig. 1: Metabolic symbiosis of tumor cells and their microenvironment (endothelial cells, neurons and glial cells). Tumors (left cell) create a metabolic niche forming gradients of secreted factors such as glutamate impacting neurons and glial factors with retrograde signals back fostering a vicious cycle in favors of tumor progression.
Our findings and those of other groups led to the consensus that gliomas actively kill brain cells. Glioma cells form a toxic micromillieu and secrete glutamate inducing neurodegeneration. Thus, brain tumors can be considered as a neurodegenerative disease as well (Savaskan et al., 2015). As a consequence, new therapeutic approaches are under investigation, i.e. metabolic normalization and neural protection.
Another important feature of brain tumor cells is their impact on the immune system. Gliomas evade the immune system and are able to switch off the brain police. We identified one key factor derived by glioma cells which is silencing the immune system.
From a clincial point of view these open questions need to be tackled:
1. Which factors form the tumor metabolome and destroy neural tissue?
2. Which epigenetic modulators can ‚prime‘ tumors and tame them?
3. How can immune competent cells be reprogrammed to fight tumor cells?
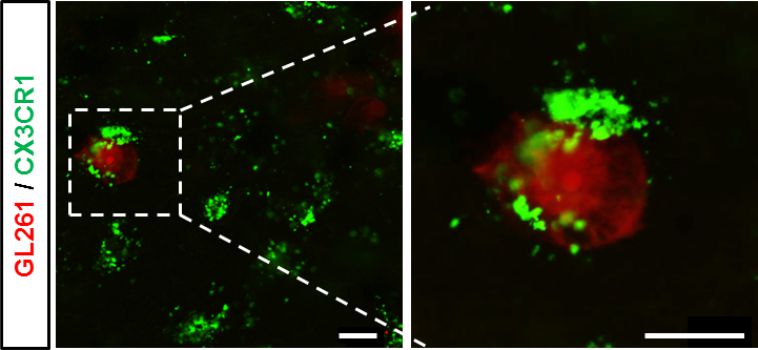
Immunocompetent cells (green) can be reprogrammed to attact tumor cells (red) and neutralize them. Apodted from Ghoochani et al., 2015.
References
Ghoochani A, Yakubov E, Sehm T, Fan Z, Buchfelder M, Eyupoglu IY, Savaskan NE,. VOGiM: a versatile ex vivo technique for analyzing tumor angiogenesis and microglia. Oncotarget. 2015; in press.
Savaskan NE, Fan Z, Buchfelder M, Eyupoglu IY. Neurodegeneration in the Brain Tumor Microenvironment: Glutamate in the Limelight. Curr Neuropharmacol. 2015;13(2):258-65.
Hatipoglu G, Hock SW, Weiss R, Fan Z, Sehm T, Ghoochani A, Buchfelder M, Savaskan NE*, Eyüpoglu IY. Sunitinib impedes brain tumor progression and reduces tumor-induced neurodegeneration in the microenvironment. Cancer Sci. 2015. doi: 10.1111/cas.12580.
Wolf I, Fan Z., Rauh M, Seufert S, Hore N, Buchfelder M, Savaskan NE*, Eyüpoglu IY,. Histone deacetylases inhibition by SAHA/Vorinostat normalizes the glioma microenvironment via xCT equilibration. Sci Rep. 2014 Sep 17;4:6226. doi: 10.1038/srep06226.
Yakubov E, Buchfelder M, Eyüpoglu IY, Savaskan NE. Selenium Action in Neuro-Oncology. Biol Trace Elem Res. 2014. [Epub ahead of print]
Sehm T; Fan Z.; Weiss R, Schwarz M, Engelhorn T, Hore N, Doerfler A, Buchfelder M, Eyüpoglu IY, Savaskan NE. The impact of dietary isoflavonoids on malignant brain tumors. CANCER MEDICINE 2014; 3(4):865-877.
Savaskan NE, Fingerle-Rowson G, Buchfelder M, Eyüpoglu IY. Brain miffed by macrophage migration inhibitory factor. Int J Cell Biol. 2012; 2012:139573.
Savaskan NE, Seufert S, Hauke J, Tränkle C, Eyupoglu IY and Hahnen E. Dissection of Mitogenic and Neurodegenerative Actions of Cystine and Glutamate in Malignant Gliomas. Oncogene 2011, 30(1):43-53.
Metabolic profiling of tumor patients
State of the art therapies for gliomas include combined multimodal approaches. However the overall survival is still fatal.
Various clinical factors have been identified with predictive power such as age, Karnofsky Performance and genetic and epigenetic predispositions.
However, the ‚first line‘ therapy of malignant gliomas consider solely the extent of tumor invasion.
We aim at defining more physical parameters such as muscle-fat ratio and fitness conditions of patients. For this we teamed up with Dr. Yurdagül Zopf, professor of internal medicine. We investigate now physical fitness, muscle index, fat status, nutritional status and metabofitness conditions and their impact on quality of life and overall survival.
We hope to identify clinical parameters, which are easy to assess and help to train patients.
Thereby, patients become fitter and more resilient for surgery and radio-chemotherapy.
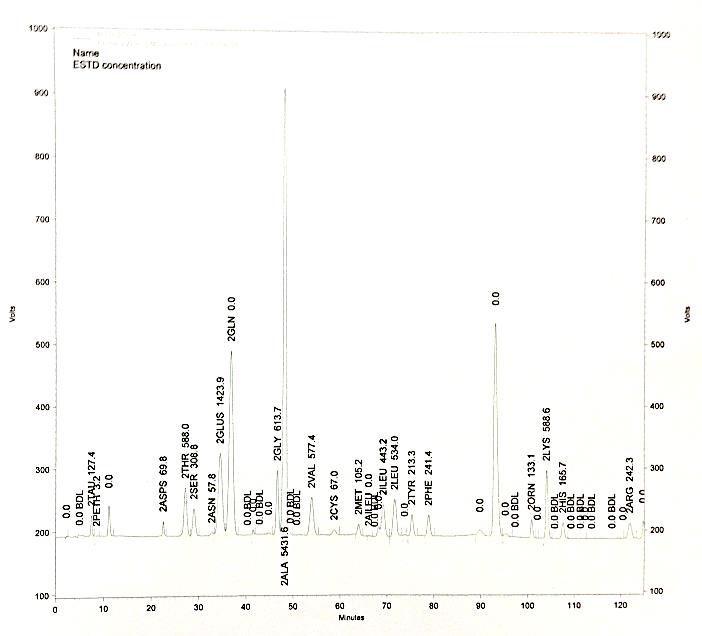
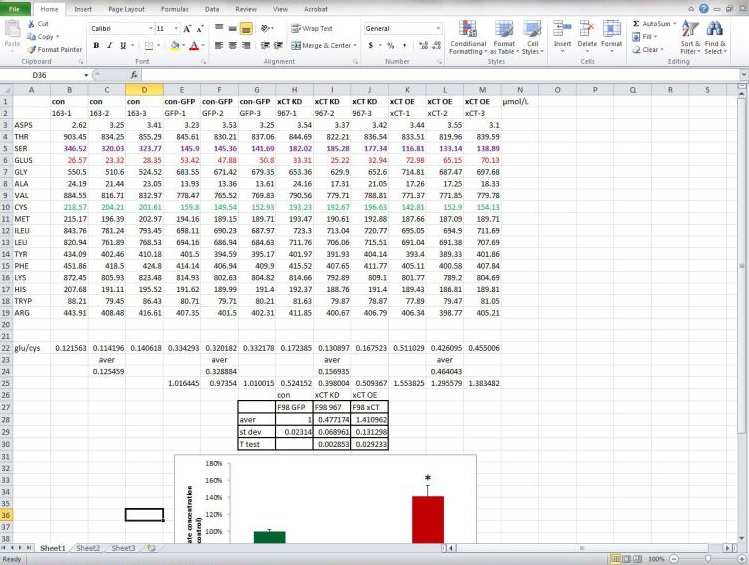
Metabolic profiling of brain tumors with quantitative approaches.
II. DiVA and novel intra-operative imaging techniques for better surgery
The extent of tumor resection is essential for patients survival and successful radio- chemotherapy.
The key for successful surgery is supra-complete tumor resection. We invented the DiVA approach: Dual intraoperative visualization approach. DiVA allows to visualize clearly tumor boarders.
Due to DiVA it is possible to resect tumors more safer and more complete.
We further develop the DiVA approach together with experts for neuronavigation and spectrometry. Thereby, we can dissolve the spatial distribution of metabolites such as N-acetyl-aspartat, cholin, glutamate, and creatine. This allows monitoring the tumor metabolism and uncovering its tumor boarders.
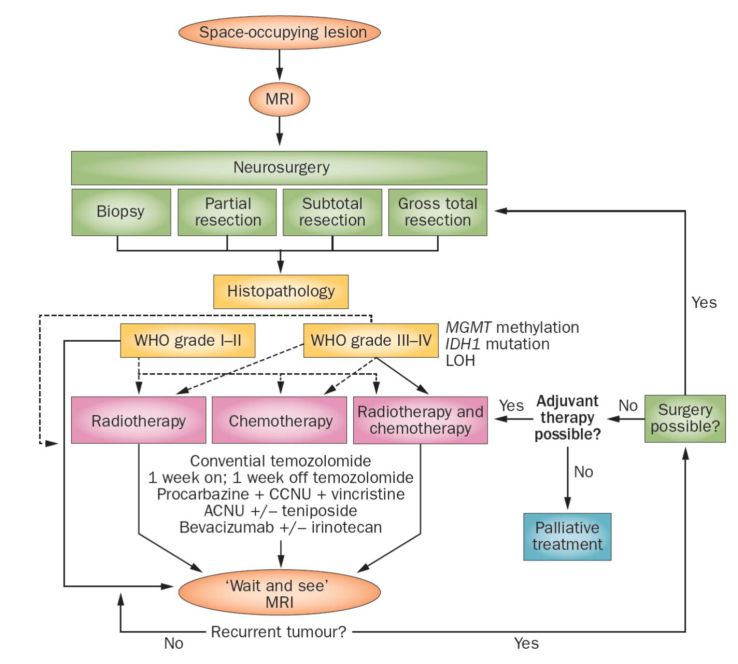
Current therapy algorhythm based on DiVA assisted neurosurgery.
References
Eyüpoglu IY, Buchfelder M, Savaskan NE. Surgical resection of malignant gliomas-role in optimizing patient outcome. Nat Rev Neurol. 2013; 9(3):141-51.
Eyüpoglu IY, Hore N, Buslei R, Fan Z, Merkel A, Buchfelder M, Savaskan NE. Intraoperative vascular DIVA (vDiVA) surgery reveals angiogenic hotspots in tumor zones of malignant gliomas. Sci Rep. 2015 Jan 22;5:7958. doi: 10.1038/srep07958.
Fan Z, Sehm T, Rauh M, Buchfelder M, Eyüpoglu IY, Savaskan NE. Dexamethasone alleviates tumor-associated brain damage and angiogenesis. PONE 2014, 9(4):e93264.
Friedlein K, Bozhkov Y, Hore N, Merkel A, Sommer B, Brandner S, Buchfelder M, Savaskan NE*, Eyüpoglu IY. A new functional classification system (FGA/B) with prognostic value for glioma patients. Sci Rep. 2015 Jul 21;5:12373. doi: 10.1038/srep12373.
III. Neural regeneration and plasticity in the brain
In the past, we put major efforts into the identification of axon growth and plasticity promoting molecules in the brain. We came up with a novel gene family including five novel genes termed Plasticty Related Genes or short PRGs (Savaskan et al., 2004). These molecules are dynamically expressed in the developing and injured brain and show diverse signaling function. In particular PRG1 is present at pre- and postsynaptic membranes (Trimbuch et al., 2009) whereas PRG3 is expressed at axon initiating segments and in the plasma membrane (Savaskan et al., 2004). Recently, we described a novel axon growth signaling pathway independent of Cdc42 and Rac. This pathway is governed by PRG5 and represents an unique feature not shared by other PRG members such as PRG1 or PRG2. Currently generation of transgenic mouse models is under way keeping us busy with testing the in vivo role of this intriguing axon growth promoting molecules (Fig. 2).
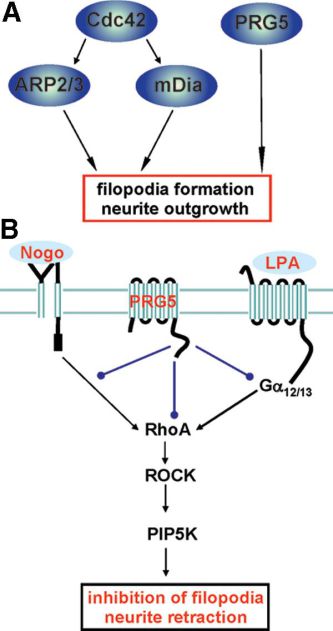
Fig. 2: PRG5 dependent axon growth signaling. A, Summury of known pathways for filopodia development and neurite growth. B, PRG5 dependent signaling at the plasma membrane.
Key publications
Nitsch R, Savaskan NE. Plasticity Related Gene 5 (PRG5) Induces Filopodia and Neurite Growth and Impedes LPA and Nogo-A Mediated Axonal Retraction.
Mol Biol Cell. 2010.
Savaskan NE, Hacke A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt C, Nimsky C, Buchfelder M, and Eyupoglu IY. siRNA mediated xCT silencing inhibits neurodegeneration and alleviates brain edema in malignant gliomas.
Nature Medicine 2008, 14 (6): 629-632.
Savaskan NE, Brauer AU, Kuhlacher M, Eyüpoglu IY, Kyriakopoulos A, Ninnemann O, Behne D, Nitsch R. Selenium deficiency increases the susceptibility to excitatory cell death.
FASEB J 2003, 17(9):1153-1155.
Brauer AU, Savaskan NE*, Kühn H, Prehn S, Ninnemann O, Nitsch R. The novel phospholipid PRG-1 is involved in axon growth and regenerative sprouting.
Nature Neurosci 2003; 6(6):572-8.
